Alleviating CRA Burdens with eClinical Innovation
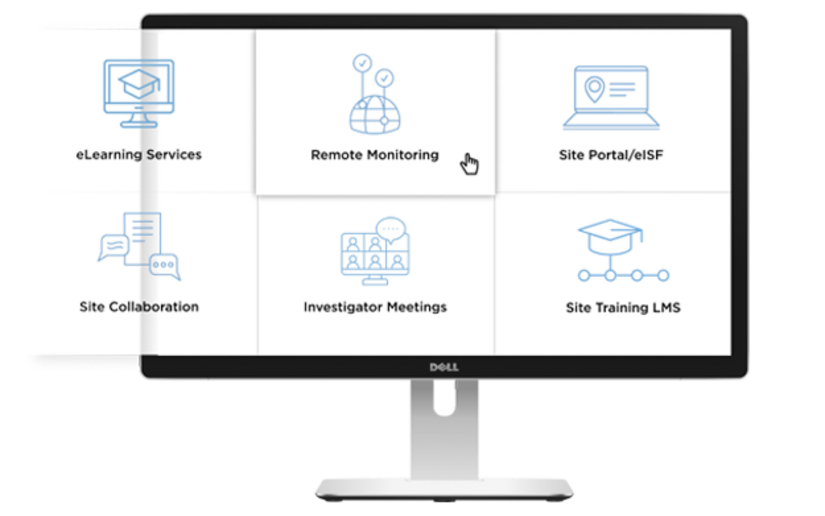
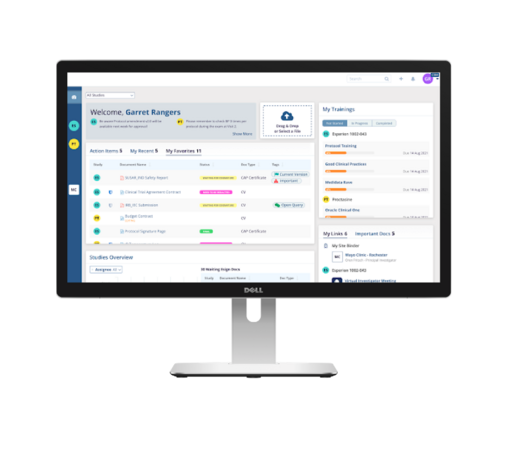
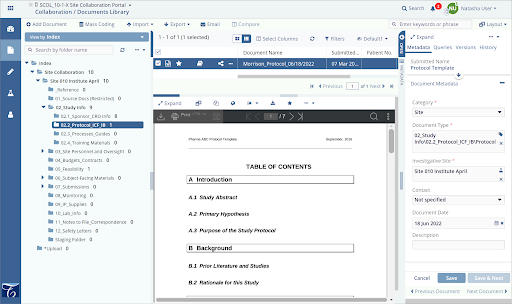
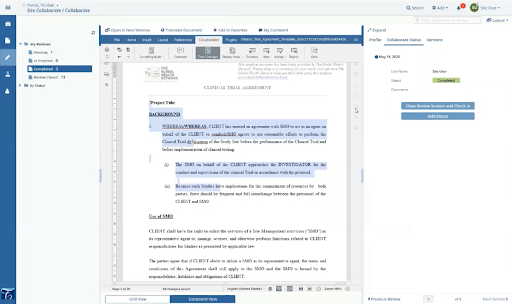
Clinical research associates (CRAs) play a crucial role in the safety and ethics of drug development. However, administrative tasks and repetitive data entry often take time away from the impactful work they do.