GET THE ULTIMATE CLINICAL TRIAL TRACKING CHECKLIST
Improve Quality and Reduce Risk in Clinical Trials

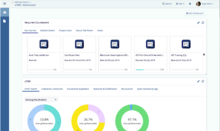
21 CFR Part 11
TI’s 21 CFR Part 11 compliant eClinical platform provides assurances for audit trail functionality, for example eTMF audit trails, electronic signatures, security and data integrity, record retention, etc.
ISO 9001:2015
TI’s QMS complies with the ISO 9001:2015 international standard and is audited in accordance to enhance customer satisfaction.
SSAE 16 SOC 2 Type 2
All data centers comply with this standard for reporting on controls at service organizations.
Annex 11, GDPR, Privacy Shield
Audited on rigorous quality standards for businesses – staffing, training, production, information management, and quality control measures.
HIPAA
TI’s policies and procedures are designed to comply with this standard and employees are required to pass introductory training.
GAMP 5
Our computerized systems are GAMP 5 compliant and are evaluated based on the framework’s risk-based approach.
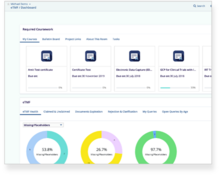
Automate and Improve Clinical Quality Assurance Processes and Management
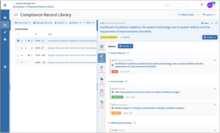
Quality assurance teams can manage standard operating procedures, policies, and any other documents from approved templates as required by the quality manual. Author, edit, approve, and manage quality documents.
Simplify quality knowledge management by connecting quality document processes to your training management.
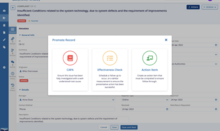
-
Approve with eSignature
-
Share Approved Document to Training Management
-
Document Becomes Effective
-
Draft from Template, Edit, and Collaborate on Document