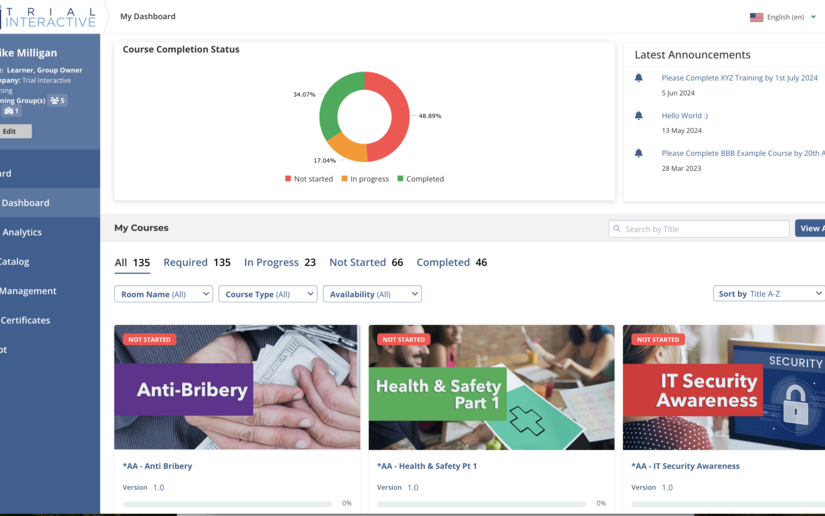
Publish Effective Documentation to the LMS and Create Training Programs
- Course Templates and Compliance Plan Builder
- Content Library and Training Catalogs
- Training Plans and Groups
- Reminder Notifications
- Training Management and Tracking
- Smart Visual Dashboards
- Compliant with Global Regulations
- eSignature Training
- Completion Verification
- Compliance Readiness Dashboard
- eTMF Interoperability for Training Records
- Content Management Interoperability