Building a Strong TMF: Compliance and Organization Essentials
Strong TMF management begins with the right foundation.
Join us at Trial Interactive’s Customer Summit: OpTImize

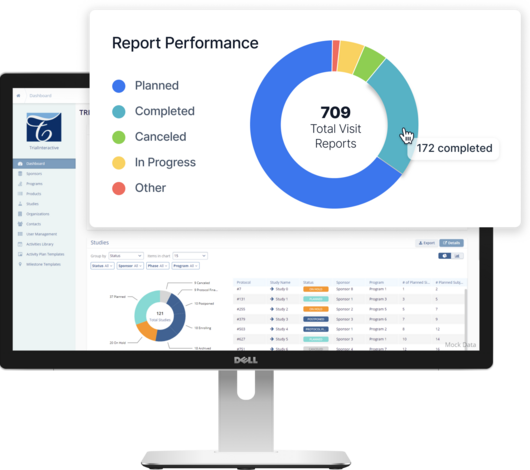
Streamline TMF Compliance with the eTMF for Growing Biotech & Pharma Companies
Our AI-powered electronic trial master file empowers every stakeholder - from study managers and CRAs, to IT teams and business leadership - to be more compliant. By streamlining TMF compliance, TI eTMF removes the friction from day-to-day clinical operations. Learn how you can ensure quality, completeness, and timeliness with an electronic trial master file.
Automate metadata and indexing workflows with machine learning to make inspection readiness easier than ever with 98% accuracy.
Launch faster with painless implementation of an eTMF system that takes weeks, not months and 24/7 support.
Streamline TMF document processing and quality control in a single source of truth for your inspection readiness and decentralized collaboration.
Developed by clinical professionals for clinical professionals, our mobile application allows CRAs to perform critical TMF tasks and oversight on the go.
Maintain TMF completeness and health with real-time document processes and audit trails.
Speed timelines and maintain TMF health with proactive oversight enabled by dashboards, analytics, and mobile insights.
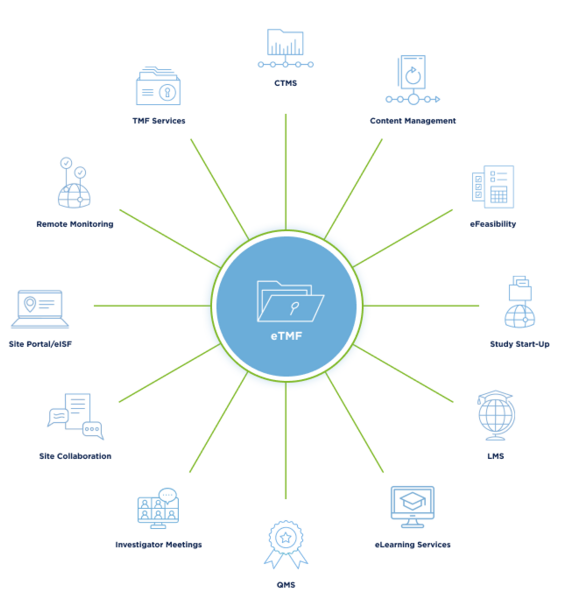
Get the support of on-demand TMF experts and document specialists. Request a Demo >
Leverage ML and AI for document processing, auto-coding, and metadata extraction. Request a Demo >
View planned and actual milestones and tasks in real-time by date, priority, and risk. Request a Demo >
Quickly and securely capture, index, and publish documents to the eTMF, directly from phone or tablet devices. Request a Demo >
Review real-time TMF insights and oversight to make faster risk-based decisions. Request a Demo >
Enable real-time document processes, version control, and 21 CFR Part 11 eSignatures. Request a Demo >
Simplify onboarding and daily use by managing just one login for all applications and modules. Request a Demo >
Stay on top of the document workstreams and maintain a complete and quality TMF. Request a Demo >
Streamline communication with an Email and Correspondence Inbox. Request a Demo >
Configure your eTMF to custom structure requirements and keep it aligned with the most current standards. Request a Demo >
All features include: QC1/QC2 Step Workflow, Quality Review Audits, Document Query and Task Management, ML Auto-Coding and Extraction, Duplicate Checks and Comparisons, Document Certification and Redaction, Flexible and compliant Metadata configuration, Required Documents and Placeholders, Country, Site, Contact Requirements, Event Manager & Health Tracking, Completeness Views, Study Start Up integration, Email and Correspondence Inbox, Bulk Upload & Migration, Responsible Party, MyTI Mobile App, CRA Reconciliation, Site and Study Collaborate / Content Authoring Request a Demo >
Automatically collect and store site feasibility responses and other essential documentation in the eTMF. Request a Demo >
Seamlessly transition from Study Start Up to eTMF. Request a Demo >
Publish content directly to the eTMF to activate real-time inspection readiness. Request a Demo >
Site and study training documentation is automatically indexed in the eTMF to show training proof to inspectors. Request a Demo >
TI eTMF connects to our full suite of Site Solutions, including eISF and Site Portal, and our QMS. Request a Demo >
Building a Strong TMF: Compliance and Organization Essentials
Strong TMF management begins with the right foundation.
The Three Pillars of Quality Management
Maintaining compliance, tracking documentation, and ensuring consistency across departments are key challenges for regulated industries. A comprehensive Quality Management System (QMS) streamlines documentation, training, and quality records management to support these needs.
Mastering Inspector Access: Streamlining Regulatory Reviews
Regulatory inspections can be complex and demanding, but a well-prepared Trial Master File (TMF) can significantly ease the process.