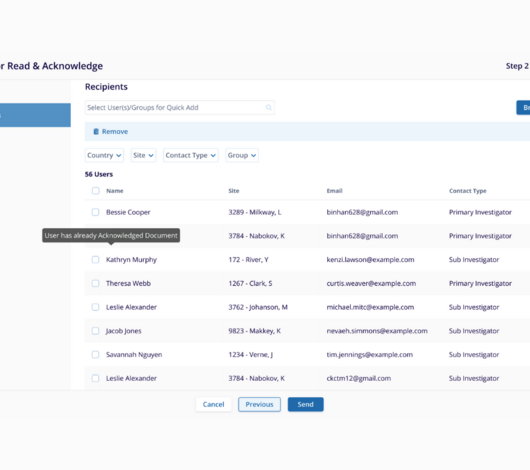
Handling safety letters effectively is critical to make sure site staff are informed about potential risks and adverse events during clinical trials. To ensure compliance with regulatory timelines and efficient communication on-site, Trial Interactive’s Safety Letter Notifications automate the distribution, tracking, and acknowledgment process.
Our solution is designed for sponsors, CROs, and sites to manage safety letters ensuring compliance, efficiency, and transparency, while minimizing manual work and the risk of errors.