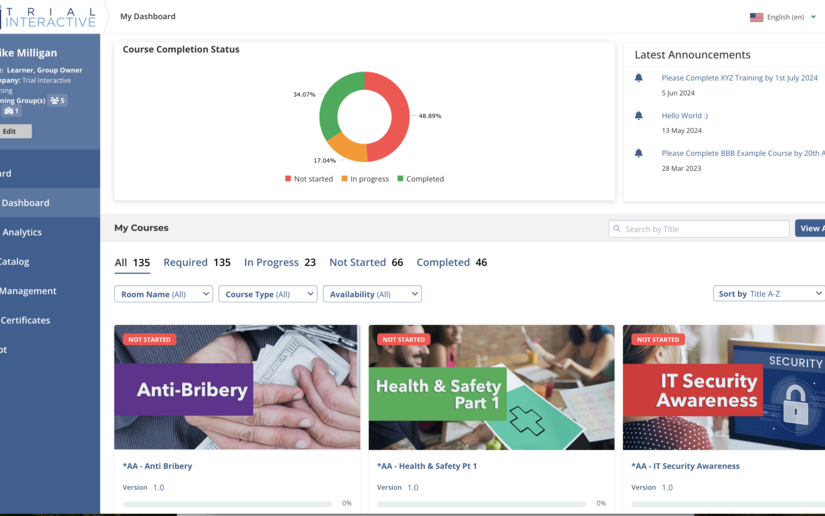
In-person training events require investigators and study coordinators to meet in one location, which requires lots of travel, logistics planning, large costs and missed hours of work, which is why only 40 - 60% of investigators are able to attend. Investigator Meetings provides an engaging, company-branded way to remotely train site personnel on clinical trial protocol and execution of study activities while reducing the amount of time, money and lost productivity usually incurred by in-person events.