GET THE ULTIMATE CLINICAL TRIAL TRACKING CHECKLIST
eFeasibility Highlights
eFeasibility tool integrated with your eTMF & 21 CFR Part 11 Complaint
-
Automated Site Scoring
-
Questionnaires Library
-
Contact Management
-
CDA Document Capture & eSignature
-
Auto-Save Capabilities
-
Question, Email, and Page Templates
-
Automated Survey Distribution & Archived Survey Data
-
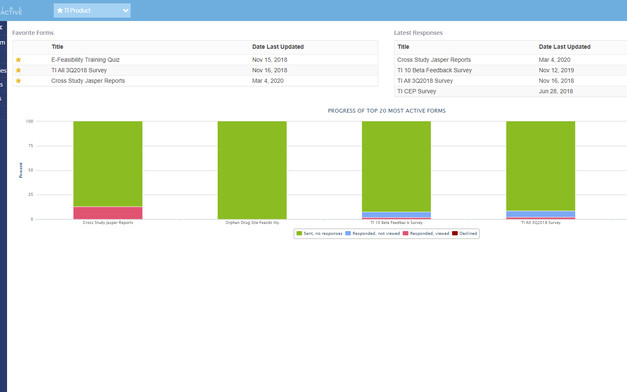
Real-Time Reports
-
Secure Cloud Hosting
A Compliant Solution for any Clinical Survey
A Site Feasibility Survey That Reduces Administrative Surveys

Start Expediting Site Selection by 35%
-
Host Site Info Securely
-
Create and Send Surveys with Ease
-
Expedite Site Selection
-
Automate Feasibility Processes
-
Get Real-Time Response Data