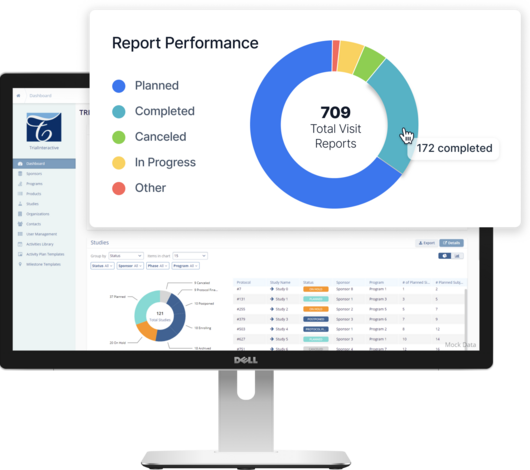
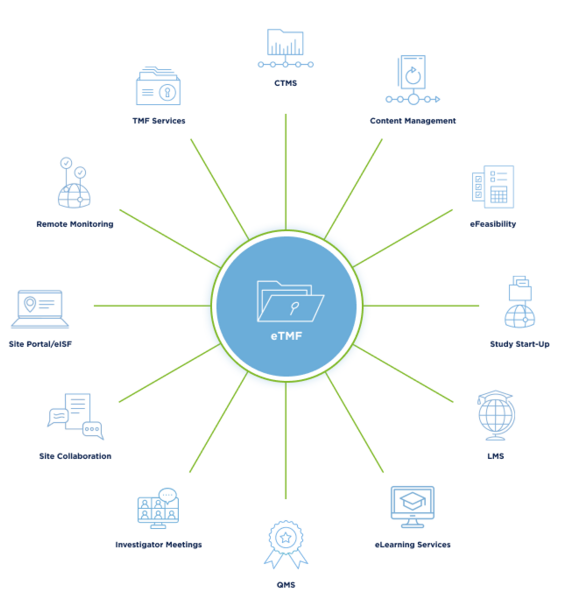
The Three Pillars of Quality Management
Maintaining compliance, tracking documentation, and ensuring consistency across departments are key challenges for regulated industries. A comprehensive Quality Management System (QMS) streamlines documentation, training, and quality records management to support these needs.