Clinical Trial Management System | Trial Interactive
Discover 360° Clinical Trial Management. Make faster risk-based decisions and reduce cycle times with TI CTMS.
Join us at Trial Interactive’s Customer Summit: OpTImize
Discover 360° Clinical Trial Management. Make faster risk-based decisions and reduce cycle times with TI CTMS.

Designed to be mobile-first, TI CTMS allows CRAs to perform critical tasks and oversight on the go.
Our CTMS is a highly configurable system that can adapt to your business processes and comes with 24/7 global support.
Our software enables study teams to avoid delays in decision making that lengthen timelines.
Mitigate compliance risks with system-level controls and a real-time audit trail that captures task, data, and correspondence.
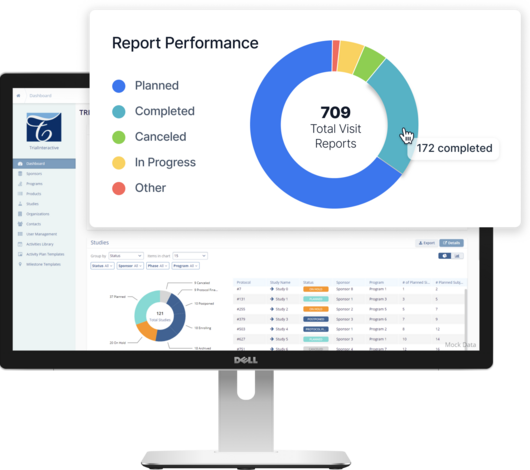
Get a single-source of truth for planning, tracking, and reporting on all your study data and documents.
Our CTMS enables remote oversight and powerful interoperability with site feasibility, study start-up, eTMF, LMS, content management, and collaboration.
Fast Implementation.
Easy to Navigate!
Unmatched Process Visibility.
Our next-generation clinical trial management system empowers every stakeholder - from CRAs and study managers, to IT teams and business leadership - to be more efficient. By simplifying study planning and oversight, TI CTMS removes the friction from day-to-day clinical operations. Learn how you can unlock real-time insights, mitigate global regulatory compliance risks, and accelerate therapeutic breakthroughs with a modern CTMS.