Join us at Trial Interactive’s Customer Summit: OpTImize
Meet the Trial Interactive Platform
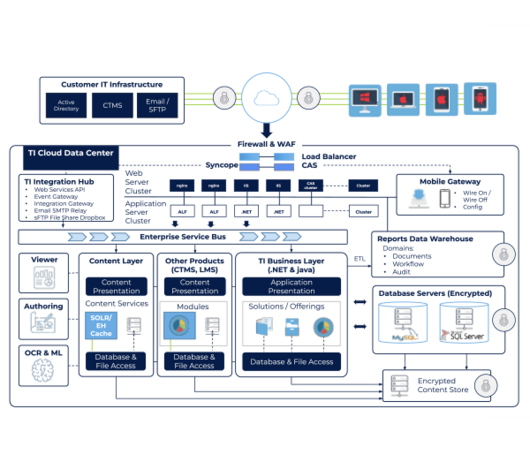
Trial Interactive’s platform is a web-based and mobile-enabled SaaS application that provides technology solutions across the full clinical trial life cycle. Our clinical and technology professionals work directly with IT teams to reduce burdens and help them meet usability, scalability, performance, validation, connectivity, and security requirements.
The TI Platform manages both content and data in a single cloud platform, and enables organizations to quickly deploy powerful solutions that streamline end-to-end processes.
Why IT Professionals Consistently Prefer Trial Interactive's Platform
See the benefits of a seamlessly connected solution.
Implementation Speed
Our eClinical solutions can be set up and brought live in as fast as 30 days, for one study or many studies.
Integration-Ready Architecture
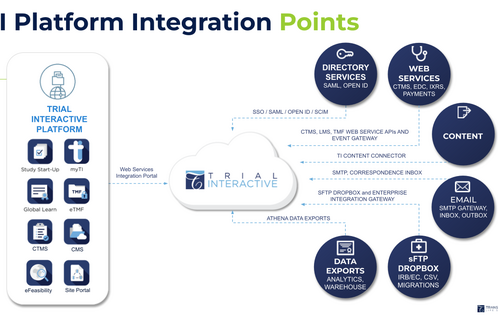
Our architecture is ready to be integrated into your IT infrastructure with a pre-validated set of TI connectors.
Global Capabilities
Our technologies scale for multi-region, multi-country clinical trials and support global regulatory compliance.
30+ Years of Life Sciences Experience
We’ve protected the integrity of therapeutics, developed 60+ SaaS products, and maintained the security of millions of documents and thousands of studies.
TI is a secure, cloud-based solution enabling real-time collaboration across your internal organization and external partners. Address security challenges of global studies and comply with global regulations. Request a Demo >
TI is a fully hosted SaaS solution in the AWS cloud environment that is 21 CFR Part 11, Annex 11, ERES, GDPR, HIPAA, ISO 9001 and GxP compliant. Request a Demo >
Reduce your implementation efforts with validation support for Trial Interactive products, solutions, integrations, and migrations. Request a Demo >
Choose flexible integration options including an API, pre-validated connectors, sFTP Dropbox, and Corporate Directory Integration with Single Sign On. Request a Demo >
Our professional and technical services teams help with whatever service requests are necessary to reduce administrative burdens and achieve your goals. Request a Demo >
Easily integrate with other systems, such as EDC or IVR, migrate data, or automate processing using the comprehensive open APIs. Request a Demo >
Online collaborative workspaces and content management rooms enable collaborative and controlled document authoring, review, and approval. Request a Demo >
Manage clinical, quality, and regulatory documents with rich capabilities including authoring, versioning, annotations, e-signatures, templates, and sharing. Request a Demo >
Automate and track business processes with configurable workstreams that are powered by adaptable, built-in machine learning features. Request a Demo >
Our mobile app works on both iOS and Android platforms, and is based on a highly secure cloud architecture. Find, access, and share documents on the go. Request a Demo >
Configure and customize dashboards and reports for KPIs, measurable metrics, simple Excel exports, and complex standardized and custom reports. Request a Demo >


