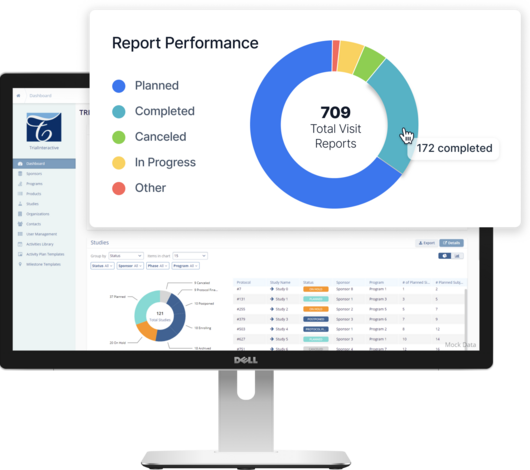
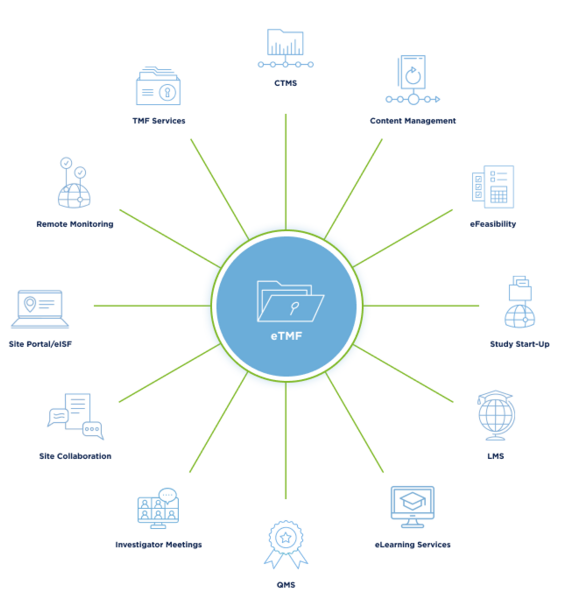
Embracing the Future: Leveraging Technology in TMF Management
Technology is rapidly transforming how Trial Master Files are managed. With advancements in artificial intelligence and cloud-based platforms, teams now have powerful tools to simplify workflows, reduce errors, and support audit readiness.